CMT Post-Procedure Cream™ 0.5 oz
CMT Post-Procedure Cream™ is the first neurocosmetic post-procedure cream proven to decrease discomfort and reduce downtime following chemical peels, microneedling and laser treatments.† This breakthrough formula features patent-pending neurocosmetic technology that helps skin recover better faster, improving the treatment experience and increasing the likelihood of completing the recommended number of treatments.
- Patent-pending neurocosmetic technology decreases discomfort post-treatment
- Helps support compromised skin by regenerating the epidermal barrier for enhanced recovery
- Reduces downtime and improves the treatment experience after the following CMT professional procedures†: Chemical (Peels), Mechanical (Microneedling, Microdermabrasion), and Thermal Energy (Fractionated CO2, Erbium, RF, IPL)
- Helps replenish and reset the skin’s native microbiome, promoting long-term skin health
- 0.5 OZ | 14 g
- 2.0 OZ | 56 g
†Consult with your healthcare professional regarding the best usage for application of this product following a professional procedure.
Who benefits? Anyone who is considering an in-office procedure but may be concerned about the length of recovery or post-treatment discomfort.
Dispense two to three pumps and apply to treated or stressed skin at least two-times daily. Reapply as needed. Consult with your healthcare professional regarding the best usage for application of this product following a professional procedure.
Patent-Pending Neurocosmetic Technology
- Helichrysum Italicum Extract
- Daucus Carota Sativa Root Extract
- Acetyl dipeptide-1- cetyl ester
- Albatrellus confluens Extract
- Opuntia Ficus-Indica Callus Culture Extract
- Saccharomyces Cerevisiae Extract
Additional powerful ingredients, including preand postbiotics, ceramides, and THD Ascorbate
Formula Information
- Dermatologist-tested
- Plastic Surgeon-tested
- Formulated for all skin types
- Fragrance-free
- Formulated without: Alcohol, Artificial Dyes, Formaldehydes, Parabens, Phthalates
- Skin-Neutral pH*
- Safe for post-procedure skin†
- Promotes a healthy microbiome
*This product is a reverse emulsion. The pH of the water phase is skin neutral (4.5 to 5.5)
†Consult with your healthcare professional regarding best usage for application of this product following a professional procedure.
Before and After*
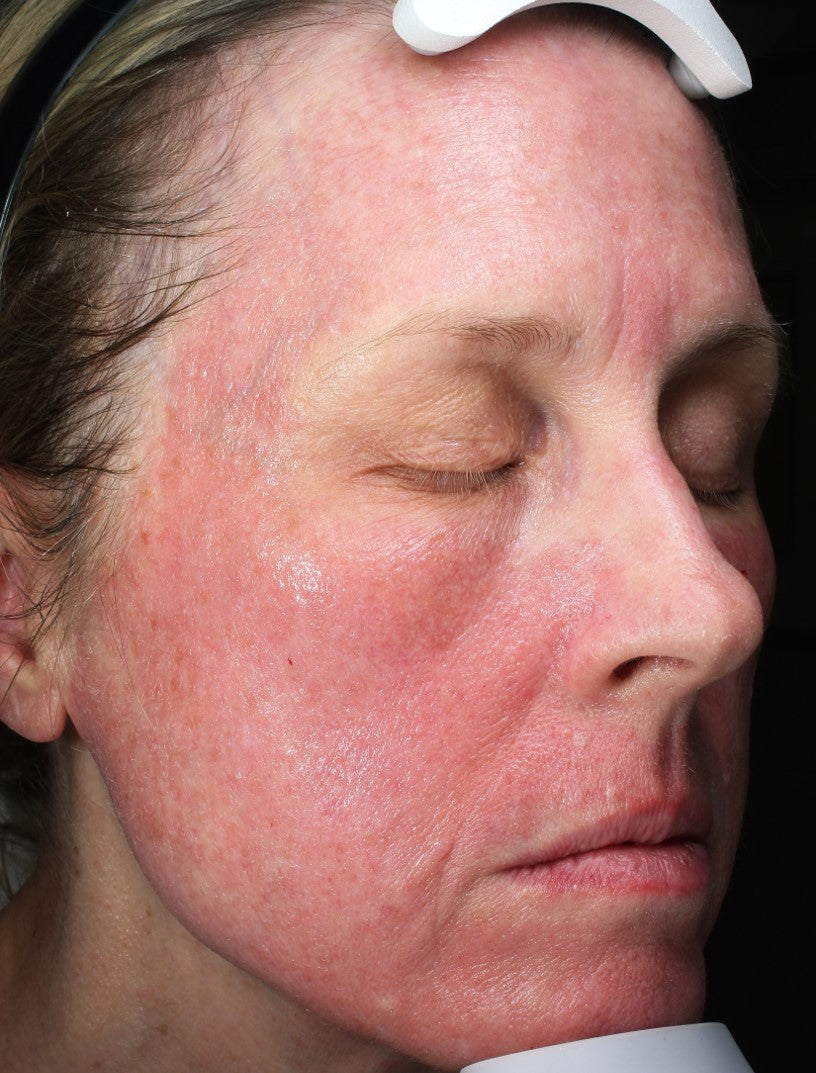
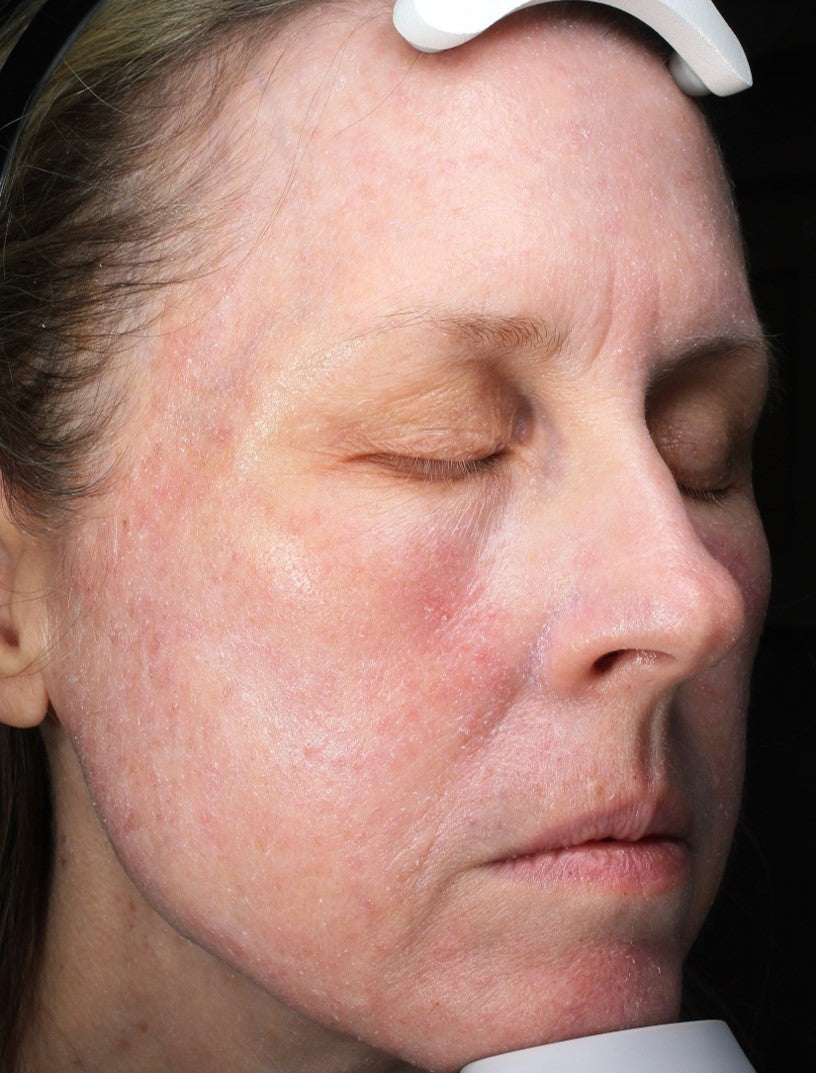
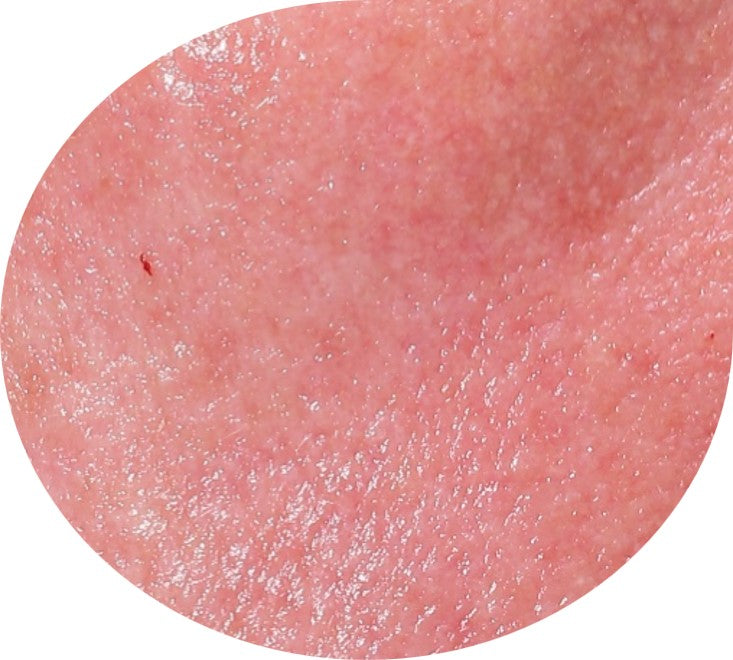
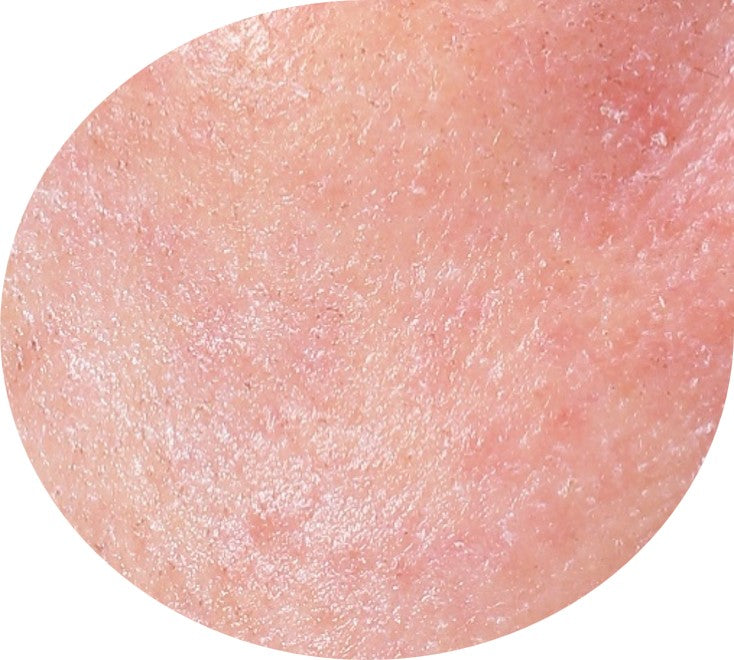
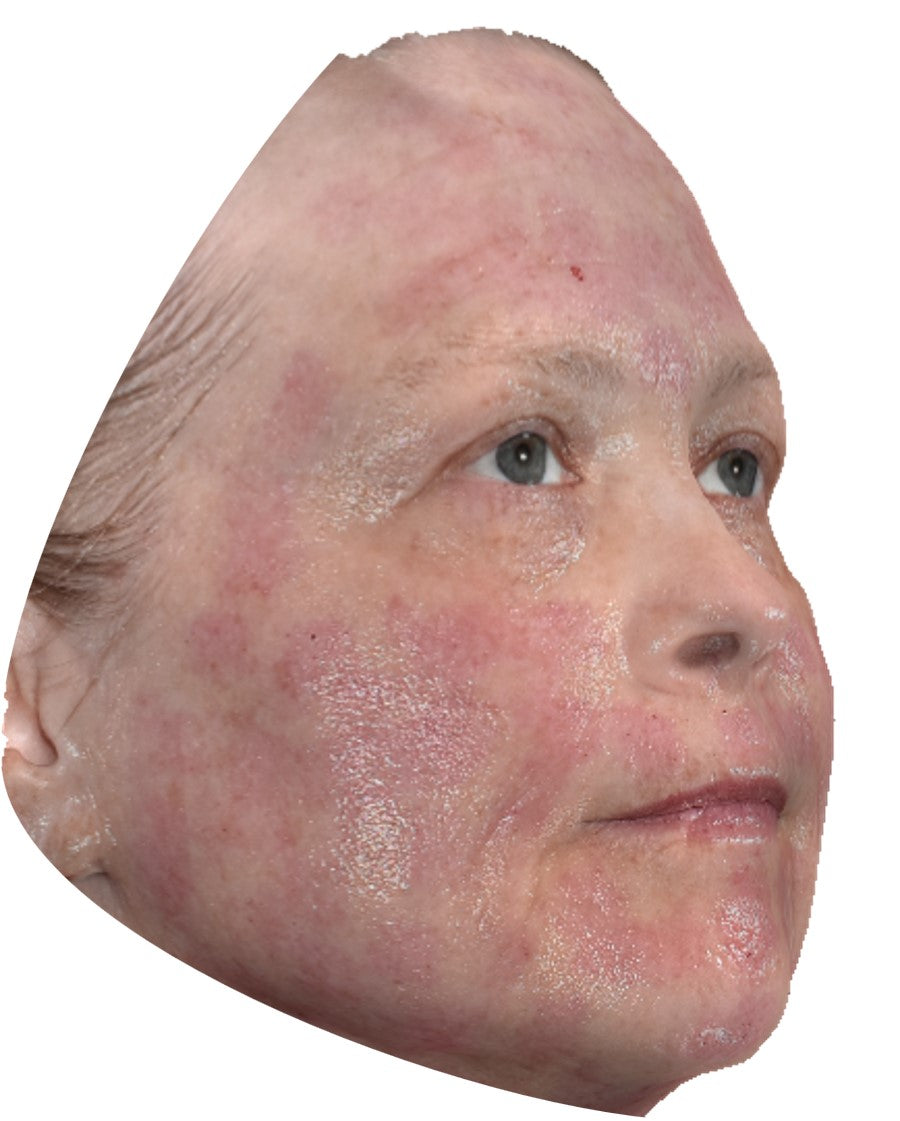
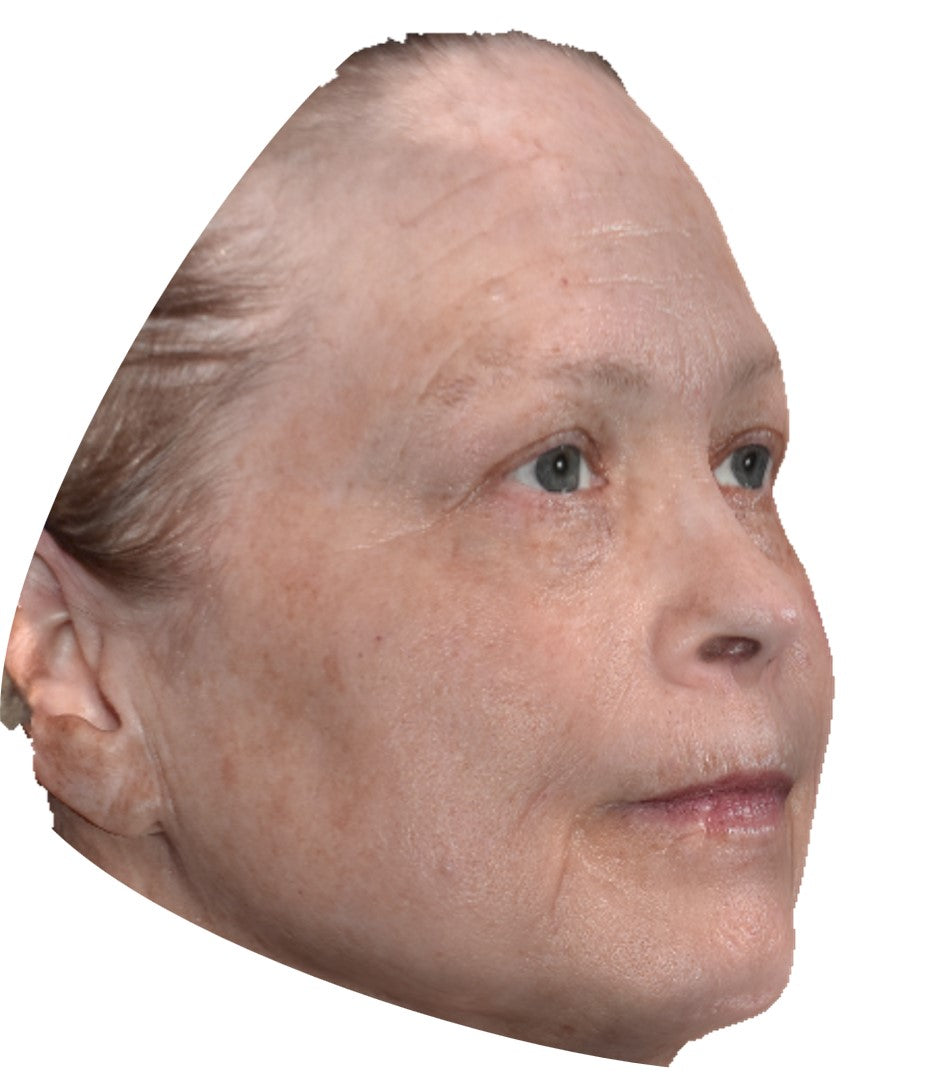
Frequently Asked Questions
What is a neurocosmetic?
-
Neurocosmetics are topical products containing unique ingredients that enhance communication between the skin and the brain. When applied to distressed skin, these products influence the perception of discomfort and produce soothing and calming effects.
How often should CMT Post-Procedure Cream™ be used after a professional procedure?
-
After a professional procedure, it is recommended that the patient discuss usage with their healthcare provider. During clinical studies, we have demonstrated relief in post-procedure discomfort by applying up to three times daily over 7 days.
How quickly does CMT Post-Procedure Cream™ help relieve discomfort?
-
During clinical studies, the product was applied after a professional treatment and was shown to relieve discomfort within 20 minutes.
Can CMT Post-Procedure Cream™ be used as a stand-alone product?
-
Yes. Although this product was only clinically tested with professional treatments, it is formulated utilizing neurocosmetic technology and additional high-potency ingredients that can provide a solution to distressed skin. For example, CMT Post-Procedure Cream™ is an effective solution for reducing discomfort when skin has been stressed by certain ingredients like Vitamin A (retinol, retinoic acid) or exposure to external aggressors (e.g., Ultraviolet Radiation and pollution).
If a patient is using a retinol product, in what order should CMT Post-Procedure Cream™ be applied?
-
It is recommended that products be applied from thinnest to thickest consistency, clearest to opaque. We recommend applying CMT Post-Procedure CreamTM after a retinol product.
How long does the relief provided by CMT Post-Procedure Cream™ last?
-
The amount of discomfort any individual perceives after a professional treatment is dependent on the individual’s pain threshold as well as the type of procedure, so how long any product can provide relief can vary. We recommend applying it at least twice a day or as needed to relieve discomfort.
How does CMT Post-Procedure Cream™ differ from a numbing cream?
-
A numbing cream is a topical local anesthetic (considered a drug) most often used pre-procedure in order to block nerve signals in the skin from reaching the brain during the procedure. CMT Post-Procedure Cream™ is used post-procedure to calm and soothe stressed skin, while also providing multiple skin health benefits, like helping support regenerating the epidermal barrier for faster recovery. Efficacy comparisons between numbing creams and CMT Post-Procedure Cream™ were not part of the clinical studies conducted.
Can CMT Post-Procedure Cream™ be used with an occlusive product?
-
Yes, it can be combined with an occlusive if needed. CMT Post-Procedure Cream™ would be applied first followed by petrolatum or other occlusive product.
How close to the eye area can CMT Post-Procedure Cream™ be applied?
-
CMT Post-Procedure Cream™ can be applied over the orbital bone of the under-eye and crow’s feet region, and not too close to the lash line. We do not recommend applying to the eyelid.
Can CMT Post-Procedure Cream ™ be used on other areas where procedures are done like the neck, decolletage, and hands?
-
CMT Post-Procedure Cream™ was clinically tested on the full face and neck. Since it was not clinically tested after procedures in other areas such as the decolletage and hands, we recommend that the patient speak to their healthcare provider.

































